Abstract
Background: When several haplo donors of similar ages are available, which factors should be prioritized remains a matter of debate. This is a frequent clinical question for recipients who are undergoing haplo hematopoietic cell transplantation (HCT) from a parent donor. Although conventionally a female donor is avoided for a male recipient due to the high risk of graft-versus-host disease (GVHD), gender-mismatch (female-to-male) has not been identified as an independent predictor of worse outcomes in the setting of T cell-replete haploidentical HCT with post-transplant cyclophosphamide (PTCy) prophylaxis. On the other hand, certain (mis)matches at specific HLA loci with haplo HCT are now being recognized as predictors of survival. Thus, it has become even more ambiguous whether HLA or non-HLA factors should be prioritized for a haploidentical donor selection. Whether HLA factors trump non-HLA factors (e.g female-to-male, donor age, relationship) remains an area of active investigation. As patient and donor ages and relationships are important predictors of outcomes and are very tightly correlated to each other, we studied the effects of HLA and non-HLA factors stratified by donor relationship (mother vs father).
Objective: We sought to compare the outcomes of T-cell replete haplo HCT using a mother vs father donor with PTCy prophylaxis, after accounting for (mis)matching at individual HLA loci and patient/donor age.
Results: We included 57 patients with a mother (n=29) vs father (n=28) donor. Median age at HCT (23 yrs vs 27 yrs, p=0.1) and donor age (50 yrs vs 52 yrs, p=0.9) were similar in both groups. Most received RIC (79% vs 86%, p=0.5), BM graft (83% vs 75%, p=0.5), had HCT-CI >3 (46% vs 64%, p=0.2), and low/intermediate DRI (45% vs 54%, p=0.8), were CMV seropositive (86% vs 79%, p=0.04), and B-leader matched (51% vs 48%, p=0.9) and ABO matched with the donor (76% vs 64%, p=0.4). The recipient was male in 59% (mother donor) vs 61% (father donor). The median follow-up was 54 vs 28 months.
Graft failure occurred in 0% vs 4%, p=0.3; the median time to neutrophil engraftment was 18 days in both groups. The cumulative incidence of grade II-IV aGVHD (65% vs 56%, p=0.3) and grade III-IV aGVHD at day 180 (31% vs 15%, p=0.2), 3-yr cGVHD (31% vs 15%, p=0.2); NRM (35% vs 26%, p=0.5), relapse (21% vs 32%, p=0.4), PFS (39% vs 36%, p=0.9) and OS was (53% vs 41%, p=0.3) in mother vs father donor, respectively.
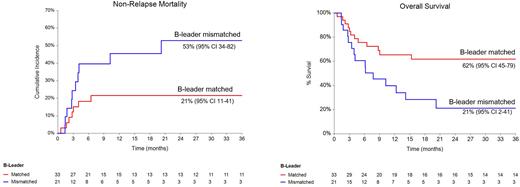
In multivariate analysis, no statistically significant differences were noted in any outcome between mother vs father donors, likely due to small number of events. However, B-leader mismatch was associated with a significantly higher risk of NRM (HR 2.7, 95% CI 1.03-7.02, p=0.04), and HLA-A mismatch was associated with a lower risk of NRM (HR 0.3, 95% CI 0.1-0.8, p=0.02), better PFS (HR 0.2, 95% CI 0.1-0.4, p<0.001) and OS (HR 0.1, 95% CI 0.05-0.4, p<0.001). HCT-CI >3 was associated with inferior PFS (HR 3.5, 95% CI 1.5-7.6, p=0.002) and OS (HR 4.1, 95% CI 1.7-9.8, p=0.002).
Conclusion: HLA factors, such as B-leader mismatch (high NRM) and HLA-A mismatch (low NRM and high PFS and OS) may have a stronger prognostic impact on mortality than donor-relationship. Recognizing the limitations of the small sample size, our data suggest that among parent haplo donors, in cases where both father and mother are B-leader matched and/or HLA-A mismatched with the recipient, father should be preferred due to a 2-fold higher risk of grade III-IV aGVHD and cGVHD (although statistically non-significant) with maternal donors. However, it remains unclear whether a B-leader mismatched (and/or HLA-A matched) father be selected over a B-leader matched (and/or HLA-A mismatched) mother. Further large-scale studies are needed to comprehend these complex immunobiological relationships.
Disclosures
Mehta:Syndax: Research Funding; Orca Bio: Research Funding. Alousi:Prolacta: Consultancy; Genetech: Consultancy; Mallinkrodt: Honoraria; Sanofi / Kadmon: Honoraria; Incyte: Honoraria, Research Funding. Kebriaei:Jazz: Consultancy; Kite: Consultancy; Ziopharm: Research Funding; Amgen: Research Funding; Pfizer: Consultancy. Nieto:Secura Bio: Research Funding; Affimed: Other: Scientific advisory Board, Research Funding; Astra Zeneca: Research Funding. Oran:AROG: Research Funding; ASTEX: Research Funding. Popat:Abbvie: Research Funding; Incyte: Research Funding; Bayer: Research Funding; Iovance: Consultancy; Novartis: Research Funding. Srour:Orca Bio: Research Funding. Rezvani:Caribou Biosciences: Other: Participates on the Scientific Advisory Board ; Navan Technologies: Other: Participates on the Scientific Advisory Board ; Bayer: Other: Participates on the Scientific Advisory Board ; GSK: Other: Participates on the Scientific Advisory Board ; Virogin Biotech: Other: Participates on the Scientific Advisory Board ; AvengeBio: Other: Participates on the Scientific Advisory Board ; GemoAb: Other: Participates on the Scientific Advisory Board ; Takeda: Patents & Royalties, Research Funding; Affimed: Research Funding. Champlin:Johnson &Johnson: Consultancy; Omeros: Consultancy; Kadmon: Consultancy; Actinium: Consultancy; General Oncology: Other: Data Safety Monitoring Board; Bluebird: Other: Data Safety Monitoring Board; Cell Source Inc.: Research Funding. Shpall:Navan: Consultancy; NY blood center: Consultancy; Affimed: Other: License agreement; axio: Consultancy; adaptimmune: Consultancy; Takeda: Patents & Royalties; Bayer: Honoraria; Fibroblasts and FibroBiologics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal